Since the discovery of the first epidermal growth factor receptor (EGFR) mutation in lung cancer in 2004, targeted therapies and immunotherapies have become a major component of the treatment arsenal for non-small cell lung cancer (NSCLC). Biomarkers are features of a cancer that predict how it will respond to certain treatments. They help doctors select the most appropriate treatment for the cancer.
Two examples of biomarkers are:
- Mutations within the cancer DNA (EGFR, ALK, ROS1, RET, etc.)
- High levels of certain proteins (e.g., PD-L1 used to decide immunotherapy)
Currently, there are FDA-approved treatment approaches for five driver mutations, and one biomarker-driven immunotherapy is also FDA-approved. For a patient to benefit from the advances of precision medicine, timely comprehensive biomarker testing (CBT) is of paramount importance to ensure that a patient gets matched to the right treatment at the right time.
We sat down with Zofia (Zosia) Piotrowska, MD, from Massachusetts General Hospital (MGH)/ Harvard Medical School to discuss the role of CBT for treatment decision making for patients with advanced-stage NSCLC. Dr. Piotrowska is a thoracic medical oncologist and clinical and translational researcher at MGH. She treats lung cancer patients and is an expert on targeted therapies. Her research focuses on improving therapies for patients with EGFR-mutant lung cancer and on developing ways to incorporate innovative diagnostic tools, specifically circulating tumor DNA (ctDNA) analyses, into clinical care.
The webinar video below offers a more detailed version of the discussion, including a section on liquid biopsies as well as the question and answer session at the end of the webinar.
What is comprehensive biomarker testing? How is it different from traditional single-analyte testing?
Traditionally, testing for biomarkers in lung cancer was done sequentially: one biomarker at a time. If a test was negative, the next test was performed. The key difference between sequential testing and CBT is that CBT usually refers to next-generation sequencing (NGS). NGS is a method to look at multiple genes in a tumor sample all at the same time. The same technology can be applied to ctDNA, or liquid biopsies. CBT helps us look for multiple mutations within a sample all at the same time. CBT may take longer to get results as compared to single tests, but that is now changing with advances in our testing capabilities. Also, CBT may turn out to be less expensive than sequential testing because multiple tests add up to increase the cost. Another advantage of CBT over single biomarker tests is that when a new target is discovered, it can be added to the comprehensive test panel given the flexibility of CBT. As an example, in 2018, the FDA approved larotectinib for NTRK-positive cancers. It was relatively easy to add this new biomarker to the NGS panel.
WHY is comprehensive biomarker testing important?
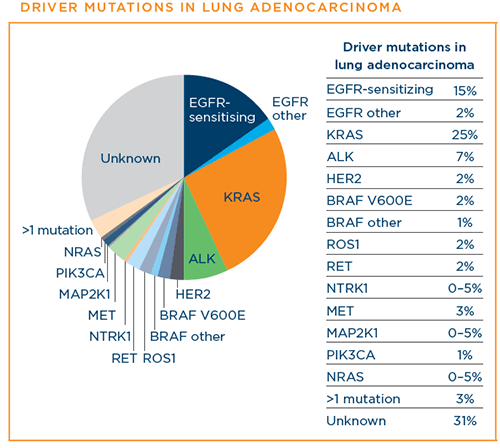
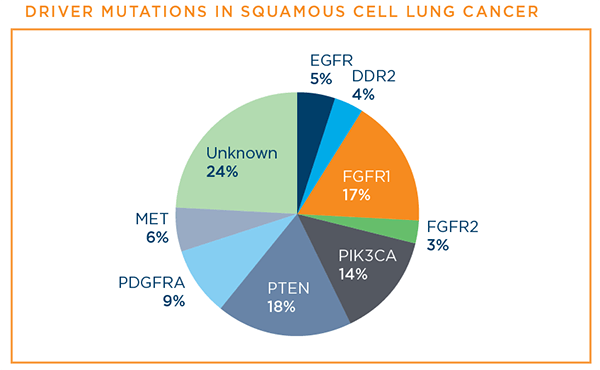
CBT helps us understand a patient's particular lung cancer on a deeper level and that helps guide doctors to the right treatment for that patient. In lung adenocarcinoma, the list of different driver mutations that can be targeted with a drug has been growing. The other common type of NSCLC is squamous cell carcinoma. Historically, we have thought about squamous cell carcinomas as cancers for which biomarker testing, or mutation testing, is less important. And what we are seeing now is that a subset of squamous cell carcinomas may have driver mutations for which there are targeted therapies, suggesting that biomarker testing in squamous cell carcinoma is important as well.
WHO should get tested? HOW is testing used to make treatment decisions?
The National Comprehensive Cancer Network® (NCCN®) guidelines state that all patients with advanced-stage NSCLC (adenocarcinoma and squamous cell lung cancer) should get their tumors tested. NCCN® is a group that generates guidelines for the treatment and management of all different types of cancers. The NCCN® guidelines are a reference that many oncologists around the country go to; insurance companies also use it to make reimbursement decisions. The NCCN® guidelines now recommend CBT—identifying the right biomarker helps get the right treatment to the right patient at the right time. CBT can help pinpoint whether a patient may qualify for an FDA-approved targeted therapy for one of the driver mutations (EGFR, ALK, ROS1, BRAF, and NTRK). In addition, CBT may also help detect additional driver mutations that may have drugs in clinical development and therefore help match a patient to a clinical trial. This is especially true for some of the rare mutations, such as MET and RET.
MET: MET exon 14 skipping mutations are seen in ~3% of adenocarcinomas. These cancers are susceptible to crizotinib. In addition, other drugs are being developed for MET mutations.
RET: RET fusions are seen as a primary mutation in ~2% of adenocarcinomas. Recently, such fusions have also been seen in EGFR-mutant lung cancers that become resistant to EGFR inhibitors. Novel RET inhibitors (LOX-292 and BLU-667) are showing promising clinical activity in clinical trials.
WHEN should a Patient with advanced-stage NSCLC get tested?
CBT should, whenever possible, be performed at the time of initial advanced-stage NSCLC diagnosis to guide therapy. If insufficient tissue is available for biomarker testing at the time of diagnosis, options to consider are to (1) send a liquid biopsy, (2) consider repeat biopsy, or (3) repeat testing later on.
In some cases, additional biomarker testing is performed at the time of progression on targeted therapies. Testing at progression helps us understand how a tumor has become resistant to a targeted therapy (mechanisms of resistance) as well as identify potential 2nd—and subsequent—lines of treatment.
For additional information, we invite you to check out our new free downloadable patient education booklet on biomarker testing.
Related Reading
Dr. Basu Roy is LUNGevity's Senior Director of Research.
