KRAS (or Kirsten rat viral oncogene homolog) is the most common driver mutationA change to the DNA of cancerous cells that caused the cancer to develop and has helped the cancer cell to grow in non-small cell lung cancer (NSCLC), accounting for 25% to 30% of all cases. Our understanding of KRAS-positive NSCLC is rapidly growing. We know that there are also many different KRAS subtypes.1
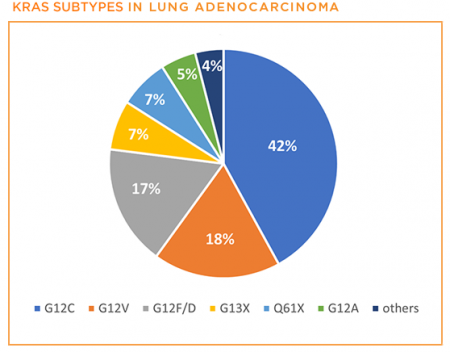
Close to half of patients with KRAS-positive NSCLC will have the G12C mutation, making it as common as EGFR-positive NSCLC. These “point” mutations within KRAS cause it to become permanently turned on in a way that helps “drive” the cancer.
How is it diagnosed?
Comprehensive biomarker testing can help determine if your lung cancer has an underlying biomarker or “driver mutation” that might make it respond to certain targeted treatments. Additionally, this testing can be used to tell what specific KRAS mutation you may have, with G12C being the most common. It is important to know this information because it will help guide you and your doctor to the most appropriate treatments for your particular form of lung cancer.
Beyond knowing which KRAS mutation you have, comprehensive biomarker testing may also identify additional mutations that are sometimes present1 and may help inform your treatment plan.
How is KRAS-positive lung cancer different from other NSCLC biomarker subtypes?
The key thing that distinguishes KRAS-positive NSCLC from other biomarker subtypes is that, while KRAS is the most common driver mutation, it has proved challenging to treat, leading many researchers to deem it “undruggable” for a long time. Until recently, chemotherapy has been considered the mainstay treatment for patients with KRAS-positive lung cancer. However, significant progress has been made in recent years, with several different types of treatments in various stages of development.2
Treatment options
Targeted Therapy
Targeted therapies are currently showing the most promise for treating KRAS-positive NSCLC. Several companies (Amgen, Mirati, Johnson & Johnson/Janssen, and others) are developing drugs specifically targeting the KRAS G12C mutation. Other companies (such as Boehringer Ingelheim and Revolution Medicines) are developing universal KRAS inhibitors that work across the different KRAS mutations that have been described, not just G12C.
Amgen’s drug, sotorasib (LumakrasTM), received FDA accelerated approval on May 28, 2021, as a second-line treatment for patients with KRAS G12C-positive NSCLC. The FDA also approved companion diagnostic tests by QIAGEN and Guardant to identify people eligible for treatment with the drug.
Common side effects with sotorasib include diarrhea, musculoskeletal pain, nausea, fatigue, liver toxicity, and cough.3
Immune Checkpoint Inhibitors
Previous studies have detected PD-L1 expression along with KRAS mutations in various KRAS NSCLC cell lines and patient samples. Immunotherapy is already a standard-of-care treatment for patients with NSCLC. Ongoing studies are now looking at whether different KRAS subtypes gain benefit from immunotherapy treatment. Additionally, several clinical trials are underway looking at combination of KRAS G12C-specific inhibitors with immune checkpoint inhibitors.
KRAS Cancer Vaccines and Engineered Immune Cells
KRAS mutations also create new epitopes or “signals” that may trigger an immune response. Researchers are currently exploring whether they can develop vaccines or engineer special immune cells (T cells or dendritic cells) that will target the tumor. Both Moderna (which developed one of the COVID-19 mRNA vaccines) and Merck are working on a KRAS mRNA vaccine. Both of these approaches (KRAS vaccines and engineered T cells) are in early stages of development.
Patient Gateway: Living with KRAS-positive lung cancer
The KRAS-Positive Patient Gateway is the central hub for updates on treatment options, research news, and patient resources designed to help people live better with KRAS-positive lung cancer.
Questions to ask your doctor
- Which KRAS mutation do I have?
- Did I receive comprehensive biomarker testing? If not, can I?
- Do I have any other co-occurring mutations that may influence my treatment?
- Am I eligible for a clinical trial?
- Am I eligible for immunotherapy?
Updated February 5, 2024
References
- Scheffler M, Ihle MA, Hein R, et al. K-ras Mutation Subtypes in NSCLC and Associated Co-occuring Mutations in Other Oncogenic Pathways. J Thorac Oncol. 2019;14:606-616. https://doi.org/10.1016/j.jtho.2018.12.013. Accessed February 5, 2024
- Salgia R, Pharaon R, Nam A, Sattler M. The improbable targeted therapy: KRAS as an emerging target in non-small cell lung cancer (NSCLC). Cell Rep Med. 2021;2:100186. https://doi.org/10.1016/j.xcrm.2020.100186. Accessed February 5, 2024.
- LUMAKRAS® (sotorasib) package insert] https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/214665s004lbl.pdf. Accessed February 5, 2024.
