Lung adenocarcinoma is a type of non-small cell lung cancer that accounts for about 40% of all lung cancers. In addition to existing treatment approaches, promising new treatment approaches are being developed.
What is lung adenocarcinoma?
Lung adenocarcinoma is a subtype of non-small cell lung cancer (NSCLC)A group of lung cancers that are named for the kinds of cells found in the cancer and how the cells look under a microscope. Lung adenocarcinoma is categorized as such by how the cancer cells look under a microscope. Lung adenocarcinoma starts in glandular cells, which secrete substances such as mucus, and tends to develop in smaller airways, such as alveoli. Lung adenocarcinoma is usually located more along the outer edges of the lungs. Lung adenocarcinoma tends to grow more slowly than other lung cancers.1,2,3,4
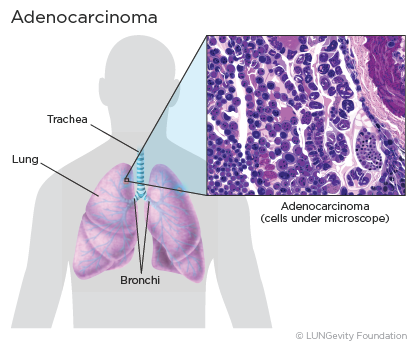
Lung adenocarcinoma accounts for 40% of all lung cancers. It is found more often in women. Younger people (aged 20-46) who have lung cancer are more likely to have lung adenocarcinoma than other lung cancers. Most lung cancers in people who have never smoked are adenocarcinomas.1,5
For more information about the signs and symptoms that might indicate lung adenocarcinoma, see the Signs & Symptoms section. Note that lung adenocarcinoma may not cause any symptoms, especially early on in its development, and that the signs and symptoms are not specific to lung adenocarcinoma and may be caused by other conditions.
Types of lung adenocarcinoma based on biomarkers
Researchers have made great progress in identifying and understanding “driver mutations” in NSCLC adenocarcinoma, which has resulted in a number of targeted therapies, a very precise type of cancer treatment that identifies and attacks specific parts of cancer cells and the signals that proteins send to cancer cells that cause them to grow and divide uncontrollably.
Genes with driver mutations for which there are FDA-approved targeted therapies for the treatment of lung cancer are listed below. Learn more about approved targeted therapies for each mutation.
- ALK
- BRAF V600E
- EGFR (including mutations not sensitive to tyrosine kinase inhibitors)
- HER2
- KRAS
- MET exon 14 skipping
- NTRK
- RET
- ROS1
You can find out more about approved treatment options for each mutation here.
Diagnosing lung adenocarcinoma
Diagnosing lung cancer is a complex process. In addition to determining whether a patient has lung cancer, diagnosing includes categorizing lung cancers in ways that help to determine the best treatment plan.
How is lung adenocarcinoma diagnosed?
Different tests are used to diagnose lung cancer and determine whether it has spread to other parts of the body. Some of these tests can also help to decide which treatments might work best. Steps and tests used in diagnosing lung adenocarcinoma include:
- Imaging tests
- Laboratory tests
- Biopsies (A tissue biopsy is the only way to confirm a diagnosis of lung cancer.)
The diagnostic approaches used for an individual will depend on medical history and condition, symptoms, location of the nodule(s), and other test results.
For more information about the different steps and tests for making a lung cancer diagnosis, see the Diagnosing Lung Cancer section.
Stages of lung cancer
Staging is a way of describing where the cancer is located, if or where it has spread, and whether it is affecting other parts of the body. Doctors use diagnostic tests to determine the cancer’s stage, so staging may not be complete until all of the tests are finished. Knowing the stage helps the doctor to recommend a treatment plan. Lung cancer is treatable at all stages.
The staging system used for lung adenocarcinoma is the TNM system, where the combination of the values assigned to a patient's cancer on three measures—T (tumor), N (node), and M (metastasis)—determine the cancer's stage.Stages range from 0 to IV. The higher the stage number, the more advanced the cancer. Only stage IV is metastatic.6,7
The Lung Cancer Staging section provides more information about how stages are determined and the characteristics of each stage.
Biomarkers
Biomarkers are features of a cancer that predict how it will respond to certain treatments. They are helpful in the determination of the most appropriate treatment for the cancer.
Biomarkers currently used in determining treatments for lung adenocarcinoma are:
- Driver mutations within the cancer DNA
- Levels of the PD-L1 protein
A patient's lung adenocarcinoma may have one of the many known driver mutations—changes in the patient's DNA that lead to lung adenocarcinoma or cause it to progress. In biomarker testing (also called mutation, genomic, or molecular testing), the patient's DNA is analyzed to determine whether any of these driver mutations is present. Nine of these driver mutations have FDA-approved therapies that specifically target them. Per National Comprehensive Cancer Network (NCCN) guidelines, it is recommended that all patients with advanced-stage NSCLC have a type of biomarker testing called comprehensive biomarker testing. Patients should discuss biomarker testing with their doctor.19
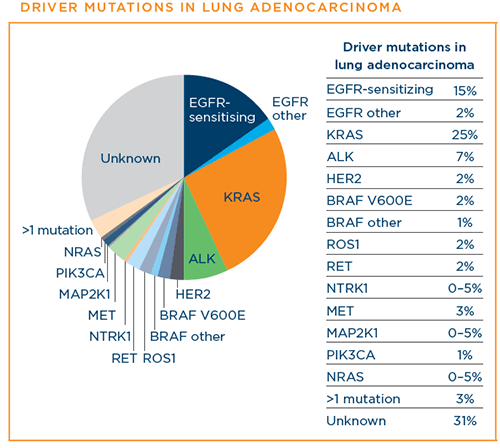
Below are the driver mutations that have been identified for lung adenocarcinoma at this time. The percentages indicate the approximate percentage of lung adenocarcinoma diagnoses that each driver mutation represents:8
The presence of a certain level of PD-L1, a protein biomarker, is used under certain conditions to determine whether a lung adenocarcinoma patient is likely to benefit from treatment with an immunotherapy drug.19
Tumor mutational burden (TMB) is the total number of mutations that result in changes in the cancer cells’ protein. TMB is used to determine whether a lung cancer patient is likely to benefit from a specific immunotherapy treatment.21,22,23
For more information about driver mutations, PD-L1, TMB, and biomarker testing, see the Biomarker Testing sections.
Treatment options for lung adenocarcinoma
There are a number of treatment options for lung adenocarcinoma. Which ones are used to treat a specific patient's lung cancer will depend on the stage of the cancer, the patient's overall health, including how well the organs of the patient's body are functioning, and the patient's preferences. A patient's age alone does not predict whether a patient will benefit from a treatment.5,7
Patients may be as involved in the treatment plan decision as they want to be. Patients should discuss all of the options, understand what the goal of each option is (for example, cure vs. control), consider the benefits and risks of each, check about likely side effects, understand how everyday life might be affected, find out what the treatment will mean financially, and not hesitate to get a second opinion if there are unaddressed concerns.17,18
It is always a good idea for a patient to take someone along to appointments to help with questions and take notes.
What are currently approved treatment options?
Approved treatment options for lung adenocarcinoma include:
- Surgery
- Radiation therapy
- Chemotherapy
- Targeted therapies
- Angiogenesis inhibitors
- Immunotherapy
In addition, new treatments are being studied for lung adenocarcinoma. These are available through clinical trials.
For more information about approved treatment options for NSCLC by stage, see the Treatment Options for Non-Small Cell Lung Cancer by Stage section.
Surgery
Surgery is a treatment option in lung cancer only when the patient is healthy enough for surgery, and the tumor:
- has been found early (is in one lung),
- is able to be completely removed safely, and
- has not spread within the chest or to other organs9,10
Surgery is the best chance for a cure. Among the types of surgery—lobectomy, wedge resection, segmentectony, pneumonectomy, and sleeve resection—lobectomy, the removal of an entire lobe, is currently considered to be the most effective.10,20
Read more about different surgical options, when they are used, and what to expect after surgery in the Treatment Options: Surgery section.
Radiation therapy
Radiation therapy is a type of cancer treatment that uses high-powered energy waves to kill cancer cells or reduce the size of tumors. It works by damaging the cancer cells' ability to multiply. Radiation only kills cancer cells directly in the path of the radiation.2,20
The type of radiation therapy most often used to treat non-small cell lung cancer, including lung adenocarcinoma, is external beam radiation therapy (EBRT), which is radiation directed at the lung cancer from outside the body. There are different types of EBRT that can be used, depending on the patient's situation.20
For more information about radiation treatment, including how it works, how and when it is given, the different kinds, and common side effects, see the Treatment Options: Radiation Therapy section.
Chemotherapy
Chemotherapy is a systemic drug treatment, most often given intravenously to lung cancer patients, that kills the rapidly growing cancer cells by traveling through the blood to reach the cancer cells wherever they are. Chemotherapy also kills rapidly growing healthy cells, which contributes to the side effects a patient may experience.
Patients whose lung cancer has spread beyond the lung to local lymph nodes are often given chemotherapy and radiation therapy. As with other types of non-small cell lung cancer, patients with lung adenocarcinoma are often given two chemotherapy agents as first-line therapy. Which drugs are chosen will depend in part on the patient’s overall health and ability to tolerate different possible side effects. For patients with lung adenocarcinoma, most often one of the platinum drugs cisplatin or carboplatin is combined with another chemotherapy drug, such as pemetrexed or docetaxel.10
For more information about chemotherapy, including how it works, how and when it is given, and possible side effects and how to manage them, see the Treatment Options: Chemotherapy section.
Targeted therapies
Targeted therapies are a type of therapy that targets cancer cells directly, rather than attacking all rapidly growing cells as chemotherapy does. Targeted therapies focus on specific parts of cells and the signals that cause cancer cells to grow uncontrollably and thrive.11 Most of the targeted therapies that have been studied and approved by the US Food and Drug Administration (FDA) belong to a class of drugs called kinase inhibitors.
As discussed earlier, there are a number of known driver mutations in lung adenocarcinoma. Targeted therapies are currently FDA-approved for patients with metastatic lung adenocarcinoma for nine of them: the anaplastic lymphoma kinase (ALK)A gene that the body normally produces but, when it fuses with another gene produces an abnormal protein that leads to cancer cell growth gene rearrangement, the epidermal growth factor receptor (EGFR)The protein found on the surface of some cells and to which epidermal growth factor binds, causing the cells to divide mutation, the HER2 mutation, the ROS1 gene rearrangement, the BRAF V600E mutation, the NTRK1 gene fusion, the mutation that leads to MET exon 14 skipping, the RET fusion, and the KRAS G12C mutation.
The biggest challenge of tyrosine kinase inhibitors (TKIs), which most targeted therapies are, is that patients with lung adenocarcinoma who initially benefit from them eventually develop resistance, known as acquired resistanceDisease progression after initial benefit with a targeted therapy. Doctors and researchers are working to overcome resistance and to keep TKIs effective for longer periods of time.12
For more information about targeted therapies, including how they work, how and when they are given, possible side effects and how to manage them, and acquired resistance, see the Treatment Options: Targeted Therapy section.
Angiogenesis inhibitors
As the body develops and grows, it makes new blood vessels to supply all of the cells with blood. This process is called angiogenesis. When the new blood vessels provide oxygen and nutrients to cancer cells, they help the cancer cells grow and spread.13
Angiogenesis inhibitors help stop or slow the growth or spread of tumors by stopping them from making new blood vessels. The tumors then die or stop growing because they cannot get the oxygen and nutrients they need. The way they do this is by blocking the cancer cells’ vascular endothelial growth factor (VEGF)A protein made by cells that stimulates new blood vessel formation receptor.13
Currently, there are two angiogenesis inhibitors FDA-approved for patients with non-small cell lung cancer, including lung adenocarcinoma.
For more information about angiogeneis inhibitors, how they work, how and when they are given, and possible side effects and how to manage them, go to the Treatment Options: Angiogenesis Inhibitors section.
Immunotherapy
Immunotherapy is a type of therapy that increases the natural ability of the patient's immune system to fight cancer. Instead of trying to stop or kill the patient's cancer cells directly, as most other cancer treatments do, immunotherapy trains the patient's own natural immune system to recognize cancer cells and selectively target and kill them.14,15
Currently, there are six FDA-approved immunotherapy drugs for patients with non-small cell lung cancer, including lung adenocarcinoma. These drugs belong to the type of immunotherapy called immune checkpoint inhibitorsAgents that target the pathways tumor cells use to evade recognition and destruction by the immune system, which work by targeting and blocking the fail-safe mechanisms of the immune system. The goal is to block the immune system from limiting itself, so the immune system can target the cancer cells.15
For more information about immunotherapy, including how the immune system works, possible side effects, other kinds of immunotherapy being studied, and questions to ask your healthcare team, see the Treatment Options: Immunotherapy section.
Finding a clinical trial that might be right for you
Clinical trials are research studies among patients to find out whether new medical approaches that are being developed are safe and effective and better than those currently being used.
In addition to the approved treatments described above, there is a great deal of promising research going on now in clinical trials focused on people with lung adenocarcinoma, including targeted therapies, immunotherapy. and new approaches in chemotherapy and radiation therapy.16 Clinical trials are an important option for patients thinking about lung adenocarcinoma treatments.
Patients considering participating in a clinical trial should start by asking their healthcare team whether there is one that might be a good match for them in their geographic area.
For more information about clinical trials and resources for finding one, see the Clinical Trials section.
Managing symptoms and side effects
Lung cancer treatments can cause side effects. Side effects from lung cancer treatment are common, but just because a side effect is common does not mean that a patient will experience it. Before treatment begins for lung adenocarcinoma, patients should discuss with their healthcare team what side effects might be expected and how to prevent or ease them. Patients should also speak with their healthcare team if and when new side effects begin, as treating them early on is often more effective than trying to treat them once they have already become severe. Although most side effects go away when treatment is over, some can last a long time.
In addition to the side effects of lung cancer treatment, lung cancer itself can result in a number of symptoms. For more information about the symptoms of lung cancer, see the Signs & Symptoms section.
Tips for managing specific symptoms and side effects related to treatment can be found in the For Supporters & Advocates section of the website, along with other practical and supportive resources for patients/survivors and caregivers.
To help reduce the severity and duration of most side effects and alleviate the cancer’s symptoms, a patient may want to request palliative care, also called “supportive care” or “symptom management.” There is sometimes confusion about the difference between palliative care and hospice care. Hospice care is a form of palliative care given only to patients whose life expectancy is six months or less. On the other hand, palliative care in general is an extra layer of support that can be initiated alongside other standard medical care. In fact, scientific evidence is starting to emerge that shows that palliative care may actually help patients live longer.
For more information about how palliative care can improve quality of life from the time of diagnosis, see the Palliative Care section.
The healthcare team
There are a number of doctors and other medical professionals who diagnose and treat people with lung cancer. Together, they make up the comprehensive medical or healthcare team that a patient sees over the course of their care. Members of the healthcare team work together to provide care for a patient, including diagnosis, treatment, and side-effect management and other support services.
For more information about what each member of a healthcare team does, see the Your Medical Team section.
Patient Gateway: Living with non-small cell lung cancer
The Non-Small Cell Lung Cancer Patient Gateway is the central hub for updates on treatment options, research news, and patient resources designed to help people live better with non-small cell lung cancer.
Updated February 10, 2024
References
- What is Non-Small Cell Lung Cancer? American Cancer Society website. https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/what-is-non-small-cell-lung-cancer.html. Revised November 20, 2023. Accessed February 5, 2024.
- NCI Dictionary of Cancer Terms. National Cancer Institute website. http://www.cancer.gov/dictionary. Accessed February 5, 2024.
- Non-Small Cell Lung Cancer Treatment (PDQ®)—Patient Version. National Cancer Institute website. https://www.cancer.gov/types/lung/patient/non-small-cell-lung-treatment-pdq. Updated October 11, 2023. Accessed February 5, 2024.
- Chen Z, Fillmore M, et al. Non-small cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014 Aug; 14(8): 535-546. doi: 10.1038/nrc3775. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5712844/. Accessed February 5, 2024.
- deGroot PM, Wu CC, et al. The epidemiology of lung cancer. Trans Lung Cancer Res. 2018 Jun; 7(3): 220233. doi: 10.21037//tlcr.2018.05.06. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6037963/. Accessed February 5, 2024.
- Lung Cancer: Metastatic—Non-Small Cell Lung Cancer. NCCN Guidelines for Patients.https://www.nccn.org/patients/guidelines/content/PDF/lung-metastatic-patient.pdf. Based on Version 3.2023 – April 13, 2023. Accessed February 5, 2024.
- Lung Cancer – Non-Small Cell: Stages. Cancer.Net website. http://www.cancer.net/cancer-types/lung-cancer-non-small-cell/stages. Approved December 2022. Accessed February 5, 2024.
- Hirsch FR, Suda K, Wiens J, Bunn PA, Jr. New and emerging targeted treatments in advanced non-small-cell-lung-cancer. Lancet. Sep 3 2016. 388(10048): 1012-1024. Accessed February 5, 2024.
- Lung Cancer: Early and Locally Advanced Non-Small Cell Lung Cancer. NCCN Guidelines for Patients. https://www.nccn.org/patients/guidelines/content/PDF/lung-early-stage-patient.pdf Version 3.2023 – April 13, 2023. Accessed February 5, 2024. Accessed February 5, 2024.
- Non-Small Cell Lung Cancer. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Version 1.2024 – December 21, 2023. Accessed February 5, 2024.
- Understanding Targeted Therapy. Cancer.Net website. https://www.cancer.net/navigating-cancer-care/how-cancer-treated/personalized-and-targeted-therapies/understanding-targeted-therapy. Approved May 2022. Accessed February 5, 2024.
- Lovly, C. Combating acquired resistance to tyrosine kinase inhibitors in lung cancer. Am Soc Clin Oncol Educ Book. 2016 FEb 1. doi: 10.14694/EdBook_AM.2015.35.e165. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4734636/. Accessed February 5, 2024.
- Angiogenesis Inhibitors. National Cancer Institute website. https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/angiogenesis-inhibitors-fact-sheet. Reviewed April 2, 2018. Accessed February 5, 2024.
- Biological Therapies for Cancer. National Cancer Institute website. https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/bio-therapies-fact-sheet. Reviewed April 26, 2018. Accessed February 5, 2024.
- Immunotherapy to Treat Cancer. National Cancer Institute website. https://www.cancer.gov/about-cancer/treatment/types/immunotherapy. Updated September 24, 2019. Accessed February 5, 2024.
- Clinicaltrials.gov. US National Institutes of Health website. http://clinicaltrials.gov. Accessed February 5, 2024.
- Making Decisions about Cancer Treatment. Cancer.Net website. https://www.cancer.net/navigating-cancer-care/how-cancer-treated/making-decisions-about-cancer-treatment. Approved October 2022. Accessed February 5, 2024.
- Cancer treatment decisions: 5 steps to help you decide. Mayo Clinic website. https://www.mayoclinic.org/diseases-conditions/cancer/in-depth/cancer-treatment/art-20047350. Updated September 13, 2022. Accessed February 5, 2024.
- Basu Roy, U. Comprehensive Biomarker Testing in Advanced-Stage Lung Cancers. Interview with Dr. Zosia Piotrowska. https://www.lungevity.org/blogs/webinar-comprehensive-biomarker-testing-in-advanced-stage-lung-cancers. Posted February 13, 2019. Accessed February 5, 2024.
- Lung Cancer - Non-Small Cell: Type of Treatment. Cancer.Net website. https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/types-treatment. Approved December 2022. Accessed February 5, 2024.
- Hellman Md, Ciuleanu T-E, et al. Nivolumab plus ipilimumab in lung cancer with a high mutational burden. N Engl J Med. 2018; 378: 2093-2104. https://www.nejm.org/doi/full/10.1056/nejmoa1801946. Accessed February 5, 2024.
- Topalian SL, Taube JM, Anders RA, Patoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016 May; 16(5): 275-287. doi: 10.1038/nrc.2016.36. https://pubmed.ncbi.nlm.nih.gov/27079802/. Accessed February 5, 2024.
- Tumor mutation burden (TMB). Caris Molecular Intelligence website. https://www.carislifesciences.com/tumor-mutational-burden/. Accessed February 5, 2024.
