Squamous cell lung cancer, also called squamous cell carcinoma of the lung, accounts for about 30% of all lung cancers. It's a type of non-small cell lung cancer (NSCLC) that typically is treated using one or more types of therapy—surgery, radiation, chemotherapy, angiogenesis inhibitors, or immunotherapy.
_0.png) To help you understand and share this information, you can request our free booklet that summarizes the detailed information in the following sections.
To help you understand and share this information, you can request our free booklet that summarizes the detailed information in the following sections.
What is squamous cell lung cancer?
Squamous cell lung cancer, or squamous cell carcinomaCancer that begins in the skin or in tissues that line or cover internal organs of the lung, is one type of non-small cell lung cancer (NSCLC)A group of lung cancers that are named for the kinds of cells found in the cancer and how the cells look under a microscope. Squamous cell lung cancer is categorized as such by how the cells look under a microscope. Squamous cell lung cancer begins in the squamous cells—thin, flat cells that look like fish scales when seen under a microscope. They line the inside of the airways in the lungs. Squamous cell lung cancer is also called epidermoid carcinomaCancer that begins in squamous cells.1,2
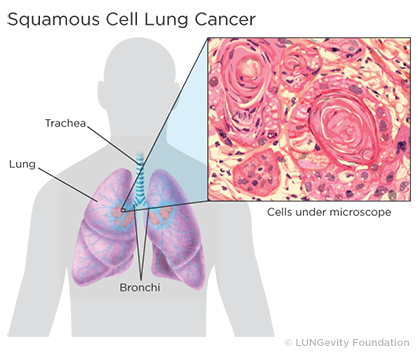
Squamous cell lung tumorsAn abnormal mass of tissue that results when cells divide more than they should or do not die when they should usually occur in the central part of the lung or in one of the main airways (left or right bronchusOne of the large air passages that lead from the trachea (windpipe) to the lungs). The tumor’s location is responsible for symptoms such as cough, trouble breathing, chest pain, and blood in the sputumMucus and other matter brought up from the lungs by coughing. If the tumor grows to a large size, a chest X-rayA type of high-energy radiation that can go through the body and onto film, making pictures of areas inside the chest, which can be used to diagnose disease or computed tomography (CT or CAT ) scanA procedure that uses a computer linked to an X-ray machine to make a series of detailed pictures of areas inside the body may detect a cavityA hollow area or hole in the lung. A cavity is a gas- or fluid-filled space within a tumor mass or noduleA growth or lump that may be malignant (cancer) or benign and is a classic sign of squamous cell lung cancer. Squamous cell lung cancer can spread to multiple sites, including the brain, spine and other bones, adrenal glands, and liver.3,4,5
About 30% of all lung cancers are classified as squamous cell lung cancer. It is more strongly associated with smoking than any other type of non-small cell lung cancer. Other risk factors for squamous cell lung cancer include age, family history, and exposure to secondhand smokeSmoke that comes from the burning of a tobacco product and smoke that is exhaled by smokers, mineral and metal dust, asbestosA group of minerals that take the form of tiny fibers, or radonA radioactive gas that is released by uranium, a substance found in soil and rock.4
For more information about the signs and symptoms that might indicate squamous cell lung cancer, see the Signs & Symptoms section. Note that squamous cell lung cancer may not cause any symptoms, especially early on its development, and that the signs and symptoms are not specific to squamous cell lung cancer and may be caused by other conditions.
Diagnosing squamous cell lung cancer
Diagnosing lung cancer is a complex process. In addition to determining whether a patient has lung cancer, diagnosing includes categorizing lung cancer in ways that help to determine the best treatment plan.
How is squamous cell lung cancer diagnosed?
Different tests are used to diagnose lung cancer and determine whether it has spread to other parts of the body. Some can also help to decide which treatments might work best. The steps and tests used in diagnosing squamous cell lung cancer include:
- Imaging tests
- Laboratory tests
- Biopsies (A tissue biopsy is the only way to confirm a diagnosis of lung cancer.)
The diagnostic approaches used for an individual will depend on medical history and condition, symptoms, location of the nodule(s), and other test results.
For more information about the different steps and tests for making a lung cancer diagnosis, see the Diagnosing Lung Cancer section.
Stages of lung cancer
Staging is a way of describing where the cancer is located, if or where it has spread, and whether it is affecting other parts of the body. Doctors use diagnostic test to determine the cancer’s stage, so staging may not be complete until all of the tests are finished. Knowing the stage helps the doctor to recommend a treatment plan. Lung cancer is treatable at any stage.
The staging system used for squamous cell lung cancer is the TNM system, where the combination of the values assigned to a patient's cancer on three measures —T (tumor), N (node), and M (metastasis)—determine the cancer's stage. Stages range from 0 to IV. The higher the stage number, the more advanced the cancer. Only stage IV is metastatic.23,24
For more information about how stages are determined and the characteristics of each stage, see the Lung Cancer Staging section.
Biomarkers
Biomarkers are features of a cancer that help predict how it may respond to certain treatments.
Biomarkers currently used in determining treatments for squamous cell lung carcinoma are:
- Driver mutations within the cancer DNA
- Levels of the PD-L1 protein
A patient's squamous cell lung cancer may have one of the known driver mutations—changes in the patient's DNA that lead to lung cancer or help it to progress. While researchers are making progress in understanding driver mutations in squamous cell lung cancer, there are no targeted therapies specifically FDA-approved yet for its treatment that require biomarker testing. However, several are being studied in clinical trials, so knowing the cancer’s biomarker profile may provide more treatment options. Per National Comprehensive Cancer Network (NCCN) guidelines, it is recommended that all patients with advanced-stage squamous cell lung cancer consider a type of biomarker testing called comprehensive biomarker testing. Patients should discuss biomarker testing with their doctor.6,27
Below are the driver mutations that have been identified for squamous cell lung cancer at this time.7
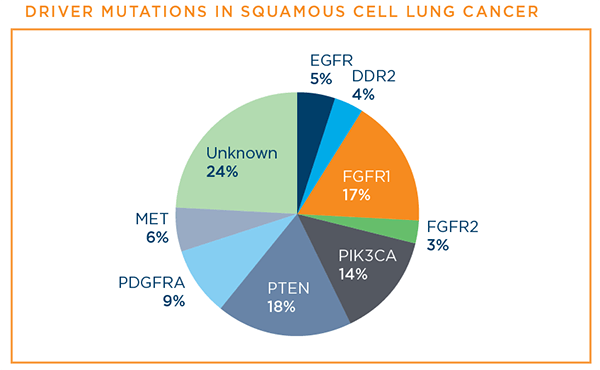
PD-L1 is a protein biomarker used to determine whether a squamous cell lung cancer patient is likely to benefit from treatment with one of the immunotherapy drugs—pembrolizumab (Keytruda®)—that has a treatment FDA-approved specifically for squamous cell lung cancer patients.6
For more information about driver mutations, PD-L1, and biomarker testing, see the Biomarker Testing, Targeted Therapy, and Immmunotherapy sections.
Treatment options for squamous cell lung cancer
Questions to discuss with your healthcare team when planning your treatment approach include:
- What are my treatment options?
- What treatment plan do you recommend for me?
- What is our goal with these treatment(s)? To eliminate my cancer? To slow its growth? To treat symptoms?
- How long will my treatment take?
- When do I need to decide on my treatment plan?
- What are the risks and potential side effects of the different treatment options?
- Will my insurance cover these treatment options?
More sets of helpful questions and checklists can be found in the For Supporters & Advocates section.
There are a number of treatment options for squamous cell lung cancer. Which ones are used to treat a specific patient’s lung cancer will depend on the stage of the cancer, the patient’s overall health, including how well the organs of the patient's body are functioning, and the patient's preferences. A patient's age alone does not predict whether a patient will benefit from a treatment.8,9
Patients may be as involved in the treatment plan decision as they want to be. Patients should discuss all of the options, understand what the goal of each option is (for example, cure vs. control), consider the benefits and risks of each, check about likely side effects, understand how everyday life might be affected, find out what the treatment will mean financially, and not hesitate to get a second opinion if there are unaddressed concerns.25,26
What Are Currently Approved Treatment Options?
Approved treatment options for squamous cell lung cancer include:
- Surgery
- Radiation therapy
- Chemotherapy
- Angiogenesis inhibitors
- Immunotherapy
In addition, new treatments are being studied for squamous cell lung cancer. These are available through clinical trials.10
For more information about approved treatment options for NSCLC by stage, see the Treatment Options for Non-Small Cell Lung Cancer by Stage section.
Surgery
Surgery is a treatment option in lung cancer only when the patient is healthy enough for surgery, and the tumor:
- has been found early (is in one lung),
- is able to be completely removed safely, and
- has not spread within the chest or to other organs11,27
Surgery is the best chance for a cure. Among the types of surgery—lobectomy, wedge resection, segmentectomy, pneumonectomy, and sleeve resection—lobectomy, the removal of an entire lobe, is currently considered to be the most effective.12,27
For more information about different surgical options, when they are used, and what to expect after surgery, see the Treatment Options: Surgery section.
Radiation therapy
Radiation therapy is a type of cancer treatment that uses high-powered energy waves to kill cancer cells or reduce the size of tumors. It works by damaging the cancer cells' ability to multiply. Radiation only kills cancer cells directly in the path of the radiation.2,12
The type of radiation therapy most often used to treat non-small cell lung cancer, including squamous cell lung cancer, is external beam radiation therapy (EBRT), which is radiation directed at the lung cancer from outside the body. There are different types of EBRT that can be used, depending on the patient's situation.12
For more information about radiation treatment, including how it works, how and when it is given, the different kinds, and common side effects, see the Treatment Options: Radiation Therapy section.
Chemotherapy
Chemotherapy is a systemic drug treatment, most often given intravenously to lung cancer patients, that kills the rapidly growing cancer cells by traveling through the blood to reach the cancer cells wherever they are. Chemotherapy also kills rapidly growing healthy cells, which contributes to the side effects a patient may experience.
Most often, the platinum-based drugs cisplatin or carboplatin are combined with another chemotherapy drug for squamous cell lung cancer treatment. An example of this is cisplatin in combination with gemcitabine.12
There is another drug approved by the FDA as first-line treatment of patients with metastatic squamous cell lung cancer. The drug, necitumumab (PortrazzaTM), is approved to be used in combination with cisplatin and gemcitabine. This drug seems to work by blocking EGFR protein expression, although no biomarker testing is necessary to determine whether an EGFR mutation is present.13,14
In addition, the tyrosine kinase inhibitor (TKI) afatinib (Gilotrif®) is FDA-approved for the treatment of patients with metastatic squamous cell lung cancer that has progressed after platinum-based chemotherapy.15
For more information about chemotherapy, including how it works, how and when it is given, and possible side effects and how to manage them, see the Treatment Options: Chemotherapy section.
Angiogenesis inhibitors
As the body develops and grows, it makes new blood vessels to supply all of the cells with blood. This process is called angiogenesis. When the new blood vessels provide oxygen and nutrients to cancer cells, they help the cancer cells grow and spread.16
Angiogenesis inhibitors help stop or slow the growth or spread of tumors by stopping them from making new blood vessels. The tumors then die or stop growing because they cannot get the oxygen and nutrients they need. The inhibitors work by blocking the cancer cells’ vascular endothelial growth factor (VEGF) receptors.16
Currently, two angiogenesis inhibitors are FDA-approved for non-small cell lung cancer, but only one of them, ramucirumab (Cyramza®), is approved for treating squamous cell lung cancer. This drug is FDA-approved in combination with the chemotherapy docetaxel for the treatment of patients with metastatic non-small cell lung cancer, including squamous cell lung cancer, whose disease has progressed after platinum-based chemotherapy.17
The other angiogenesis inhibitor, bevacizumab (Avastin®), is not an option for squamous cell lung cancer because of the risk of pulmonary hemorrhage.18,19
For more information about angiogenesis inhibitors, how they work, how and when they are given, and possible side effects and how to manage them, see the Treatment Options: Angiogenesis Inhibitors section.
Immunotherapy
Immunotherapy is a type of therapy that increases the natural ability of the patient's immune system to fight cancer. Instead of trying to stop or kill the patient's cancer cells directly, as most other cancer treatments do, immunotherapy trains the patient's own natural immune system to recognize cancer cells and selectively target and kill them.20,21
Currently, there are four FDA-approved immunotherapy drugs for patients with non-small cell lung cancer, including squamous cell lung cancer. These drugs belong to the type of immunotherapy called immune checkpoint inhibitors, which work by targeting and blocking the fail-safe mechanisms of the immune system. The goal is to block the immune system from limiting itself, so the immune system can target the cancer cells.21
Note: It’s important to let your healthcare team know if you are experiencing any problems while on treatment, so they can sort out whether the problems are related to treatment or not. It is also important to let the team know if you have a history of an autoimmune disease. It is possible that immunotherapies may make autoimmune diseases worse.
For more about immunotherapy, how the immune system works, treatments, possible side effects, other kinds of immunotherapy being studied, and questions to ask your healthcare team, see the Treatment Options: Immunotherapy section.
Finding a clinical trial that might be right for you
Clinical trials are research studies among patients to find out whether new medical approaches that are being developed are safe and effective and better than those currently being used.
In addition to the approved treatments described above, there is a great deal of promising research going on now in clinical trials focused on people with squamous cell lung cancer, including targeted therapies (a good example of a targeted therapies clinical trial that includes squamous cell lung cancer patients is the Lung-MAP clinical trial22), immunotherapy, and new approaches in chemotherapy and radiation therapy.10 Clinical trials are an important option for patients thinking about squamous cell lung cancer treatments. Patients considering participating in a clinical trial should start by asking their healthcare team whether there is one that might be a good match for them in their geographic area.
For more information about clinical trials and resources for finding one, see the Clinical Trials section.
Managing symptoms and side effects
Lung cancer treatments can cause side effects. Side effects from lung cancer treatment are common, but just because a side effect is common does not mean that a patient will experience it. Before treatment begins for squamous cell lung cancer, patients should discuss with their healthcare team what side effects might be expected and how to prevent or ease them. Patients should also speak with their healthcare team if and when new side effects begin, as treating them early on is often more effective than trying to treat them once they have already become severe. Although most side effects go away when treatment is over, some can last a long time.
In addition to the side effects of lung cancer treatment, lung cancer itself can result in a number of symptoms. For more information about the symptoms of lung cancer, see the Signs & Symptoms section.
Tips for managing specific symptoms and side effects related to treatment can be found in the For Supporters & Advocates section of the website, along with other practical and supportive resources for patients/survivors and caregivers.
To help reduce the severity and duration of most side effects and alleviate the cancer’s symptoms, a patient may want to request palliative care, also called “supportive care” or “symptom management.” There is sometimes confusion about the difference between palliative care and hospice care. Hospice care is a form of palliative care given only to patients whose life expectancy is six months or less. On the other hand, palliative care in general is an extra layer of support that can be initiated alongside other standard medical care. In fact, scientific evidence is starting to emerge that shows that palliative care may actually help patients live longer.
For more information about how palliative care can improve quality of life from the time of diagnosis, see the Palliative Care section.
The healthcare team
There are a number of doctors and other medical professionals who diagnose and treat people with lung cancer. Together, they make up the comprehensive medical or healthcare team that a patient sees over the course of their care. Members of the healthcare team work together to provide care for a patient, including diagnosis, treatment, side-effect management, and other support services.
For more information about what each member of the healthcare team does, see the Your Medical Team section.
Patient Gateway: Living with non-small cell lung cancer
The Non-Small Cell Lung Cancer Patient Gateway is the central hub for updates on treatment options, research news, and patient resources designed to help people live better with non-small cell lung cancer.
Updated February 5, 2024
References
- Non-Small Cell Lung Cancer Treatment (PDQ®): General Information About Non-Small Cell Lung Cancer. National Cancer Institute website. http://www.cancer.gov/types/lung/patient/non-small-cell-lung-treatment-pdq Updated October 11, 2023. Accessed February 5, 2024.
- NCI Dictionary of Cancer Terms. National Cancer Institute website. http://www.cancer.gov/dictionary. Accessed February 5, 2024.
- Wistuba I, Brambilla E, Noguchi M. Chapter 17. Classic Anatomic Pathology and Lung Cancer. In: Pass HI, Ball D, Scagliotti GV, eds. IASLC Thoracic Oncology, Second Edition. Philadelphia, PA: Elsevier. 2018: 143-163.
- Squamous cell carcinoma of the lung. Harvard Health Publishing website. https://www.health.harvard.edu/cancer/squamous-cell-carcinoma-of-the-lung. Updated March 31, 2023. Accessed February 5, 2024.
- Gill R, Matsusoka S, Hatabu H. Cavities in the Lung in Oncology Patients: Imaging Overview and Differential Diagnoses. Applied Radiology website. https://appliedradiology.com/articles/cavities-in-the-lung-in-oncology-patients-imaging-overview-and-differential-diagnoses. Posted June 9, 2010. Accessed February 5, 2024.
- Basu Roy, U. Comprehensive Biomarker Testing in Advanced-Stage Lung Cancers. Interview with Dr. Zofia Piotrowska. https://www.lungevity.org/blogs/webinar-comprehensive-biomarker-testing-in-advanced-stage-lung-cancers. Posted February 13, 2019. Accessed February 5, 2024.
- Hirsch FR, Suda K, Wiens J, Bunn PA Jr. New and emerging targeted treatments in advanced non-small cell lung cancer. Lancet. Sep 3 2016. 388(10048): 1012-1024. Accessed February 5, 2024.
- Lung Cancer - Non-Small Cell: Stages. Cancer.Net website. https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/stages. Approved December 2022. Accessed February 5, 2024.
- Lung Cancer. Mayo Clinic website. https://my.clevelandclinic.org/health/diseases/4375-lung-cancer#management-and-treatment. Reviewed October 31, 2022. Accessed February 5, 2024.
- Clinicaltrials.gov. US National Institutes of Health website. http://clinicaltrials.gov. Accessed February 5, 2024.
- Lung Cancer: Early and Locally Advanced—Non-Small Cell Lung Cancer. NCCN Guidelines for Patients. https://www.nccn.org/patients/guidelines/content/PDF/lung-early-stage-patient.pdf Based on Version 3.2024 – April 13, 2023. Accessed February 5, 2024.
- Lung Cancer - Non-Small Cell: Types of Treatment. Cancer.Net website. https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/types-treatment. Approved December 2022. Accessed February 5, 2024.
- Email exchange with Paul Paik, MD, Memorial Sloan Kettering. February 11, 2021.
- PortrazzaTM (necitumumab) injection [package insert]. 2015. Eli Lilly and Company, Indianapolis, IN. http://pi.lilly.com/us/portrazza-uspi.pdf. Revised November 2015. Accessed February 5, 2024.
- Gilotrif® (afatinib) tablets [package insert]. 2013. Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/201292s017lbl.pdf. Revised April 2022. Accessed February 5, 2024.
- Angiogenesis Inhibitors. National Cancer Institute website. https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/angiogenesis-inhibitors-fact-sheet. Reviewed April 2, 2018. Accessed February 5, 2024.
- Cyramza® (ramucirumab) injection [package insert]. 2014. Eli Lilly and Company, Indianapolis, IN. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125477s042lbl.pdf. Revised March 2022. Accessed February 5, 2024.
- Avastin® (bevacizumab) injection [package insert]. 2014. Genentech, Inc., South San Francisco, CA. http://www.gene.com/download/pdf/avastin_prescribing.pdf. Revised January 2021. Accessed February 5, 2024.
- Reck M, Barlesi F, et al. Predicting and managing the risk of pulmonary haemorrhage in patients with NSCLC treated with bevacizumab: a consensus report from a panel of experts. Annals of Oncology. 23: 1111-1120, 2012. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3335247/. Posted November 4, 2011. Accessed February 5, 2024.
- Biological Therapy for Cancer. Mayo Clinic website. https://www.mayoclinic.org/tests-procedures/biological-therapy-for-cancer/about/pac-20385261. Copyright 2021. Accessed February 5, 2024.
- Immunotherapy to Treat Cancer. National Cancer Institute website. https://www.cancer.gov/about-cancer/treatment/types/immunotherapy. Updated September 24, 2019. Accessed February 5, 2024.
- Lung-MAP. The Lung-MAP website. https://www.lung-map.org/. Accessed February 5, 2024.
- Lung Cancer: Metastatic—Non-Small Cell Lung Cancer. NCCN Guidelines for Patients. https://www.nccn.org/patients/guidelines/content/PDF/lung-metastatic-patient.pdf. Based on Version 3.2023 – April 13, 2023. Accessed February 5, 2024.
- Lung Cancer — Non-Small Cell: Stages. Cancer.Net website. http://www.cancer.net/cancer-types/lung-cancer-non-small-cell/stages. Approved December 2022. Accessed February 5, 2024.
- Making Decisions about Cancer Treatment. Cancer.Net website. https://www.cancer.net/navigating-cancer-care/how-cancer-treated/making-decisions-about-cancer-treatment. Updated October 2022. Accessed February 5, 2024.
- Cancer treatment decisions: 5 steps to help you decide. Mayo Clinic website. https://www.mayoclinic.org/diseases-conditions/cancer/in-depth/cancer-diagnosis/art-20044544. Updated April 25, 2019. Accessed February 5, 2024.
- Non-Small Cell Lung Cancer. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Version 1.2024 – December 21, 2023. Accessed February 5, 2024.
