Read time: 3 minutes.
Timely comprehensive biomarker testing has become the cornerstone of precision medicine in lung cancer care. For people diagnosed with non–small cell lung cancer (NSCLC), the ability to quickly identify specific genetic drivers can mean the difference between receiving a targeted, effective therapy, and starting a less personalized treatment.
A new study led by the LUNGevity Foundation, in partnership with the Association for Molecular Pathology and the American Society for Clinical Pathology, examines how current Centers for Medicare and Medicaid Services (CMS) policies are unintentionally delaying this process and what can be done to fix it.
The study, presented at the ASCO Quality Care Symposium 2025, addresses critical challenges in CMS regulations, including the “14-Day Rule,” and uncertainty over who can order molecular tests, that often prevent pathologists from initiating biomarker testing as part of the diagnostic workflow.
“When testing is delayed because of administrative hurdles, patients may start treatment before critical biomarker results are available,” explains researcher Nikki Martin, Senior Director of Precision Medicine Initiatives at LUNGevity Foundation. “That can limit their options and potentially impact outcomes.”
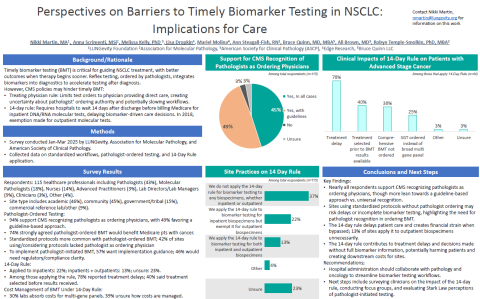
To better understand these barriers, the team surveyed 115 healthcare professionals across the United States in early 2025. The results revealed overwhelming support for empowering pathologists to order biomarker testing directly, with 94% of respondents agreeing CMS should recognize pathologists as ordering physicians, and nearly half of respondents wanting this process formalized through professional society guidelines.
Survey findings underscored the urgency of policy changes. One of the topics that was probed is the so-called 14-day rule, a CMS rule that affects how lab tests are billed when tissue originates from the hospital setting, which can unfortunately delay biomarker (or molecular) test ordering by two weeks (hence the 14-day euphemism).
Of the respondents who said they applied the 14-Day Rule to tissue samples identified for biomarker testing, 78% noted the 14-Day Rule caused treatment delays, and 40% reported that treatment decisions were sometimes made before results were received.
Results also suggest there is significant variability in how the rule is applied across sites. Some hospitals do not apply the rule at all, meaning there was no delay in ordering tests, while other hospitals only apply it to outpatients or inpatients. Some hospitals apply the rule to tissue samples (regardless of whether the patient is an outpatient or inpatient) when the tissue is acquired.
These data highlight a systemic problem. Policies designed decades ago for billing and compliance are not in step with the advancements of precision medicine where delays like these mean lives are impacted.
Nikki Martin, Senior Director of Director of Precision Medicine Initiatives at LUNGevity Foundation
The next phase of this work will involve gathering broader feedback from pathologists, oncologists, and hospital billing teams to develop concrete policy recommendations for CMS.
In partnership with numerous experts and professional societies, a separate research team is also exploring best practices for implementing “reflex” testing protocols, which is when biomarker testing is automatically ordered by the pathologist or another multi-disciplinary team member after a cancer diagnosis. The research team is developing a solutions roadmap for sites with different approaches for implementing reflex testing.
Ultimately, this research, in conjunction with research and advocacy efforts led by other organizations like the ACS National Lung Cancer Roundtable, could reshape how biomarker testing is integrated into cancer diagnostics. By recognizing pathologists as key decision-makers and eliminating unnecessary administrative barriers, healthcare systems can reduce delays, improve equity, and ensure patients receive the right treatment faster.
“Our goal,” Martin emphasizes, “is to champion reflex testing so patients spend less time waiting and more time getting the care they need."