Read time: 2 minutes.
Comprehensive biomarker testing is one of the most powerful tools in modern lung cancer care, guiding treatment decisions and improving patient outcomes. Yet despite clear clinical guidelines, implementation remains inconsistent across health systems. A new study led by LUNGevity Foundation in collaboration with the Association for Molecular Pathology and the American Society for Clinical Pathology (ASCP) sheds light on how biomarker testing is actually being conducted—and where the biggest barriers lie.
Although biomarker testing is recommended for all people with advanced cancer, many do not receive it in time to guide their initial treatment.
“We know that timely and comprehensive testing can be life-changing for patients,” says Nikki Martin, Senior Director of Precision Medicine Initiatives at LUNGevity. “But we also know that delays or incomplete testing can mean missed opportunities for targeted therapies.”
Limited data on how testing is ordered and processed makes it difficult to identify where breakdowns occur.
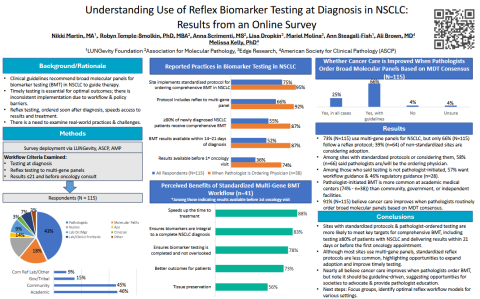
To better understand the current landscape, researchers conducted a national survey of more than 100 professionals involved in biomarker testing. The study explored:
- How and when tests are ordered
- Whether reflex testing to multi-gene panels is standard practice
- Whether results are available before patients meet with their oncologists
“Anyone involved in cancer testing has seen firsthand that timely and consistent biomarker testing still isn’t standard everywhere,” says Martin. “What’s different about this study is that it puts real numbers behind what’s been intuitively understood for years—showing, in data, the gaps and barriers that patients are still facing.”
The results—presented at ASCP’s annual meeting—revealed a significant gap in standardization. More than half of respondents reported either having no formal biomarker testing protocol or one that did not meet established best-practice criteria. Even among institutions with protocols, barriers such as reimbursement challenges, prior authorization, and the “14-day rule” regulation were identified as major obstacles.
“These policies can unintentionally delay care,” Martin explains. “We need to ensure that regulations support—not hinder—timely testing.”
Ultimately, this work could fuel advocacy initiatives that lead to major changes in how biomarker testing is implemented nationwide. By first identifying and then addressing these barriers, we can build cancer care systems that empower patients by providing faster access to the information that drives the most effective, personalized treatments. “Every delay in testing is a delay in the right treatment,” says Martin. “Fixing that can save lives.”